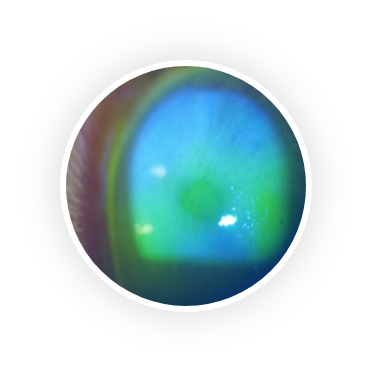
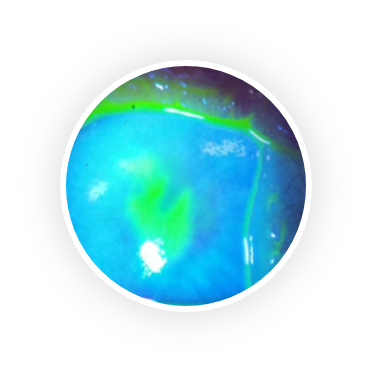
Dry eye syndrome (DES) is one of the most frequently encountered eye conditions seen by eye care practitioners. 25% of patients who visit ophthalmic clinics report symptoms of dry eye and the prevalence of DES is estimated to be 7.4% to 33.7%, making it a growing public health problem.
DES has two distinct components: insufficient tear production and tear evaporation. Insufficient tear production is caused by Sjögren’s Syndrome, an autoimmune disease that attacks the lacrimal (tear) gland. Tear evaporation is caused by insufficient lipid production by the Meibomian gland, lipids that constitute the top layer of the tear film. Whatever the cause, there is almost always a component of inflammation and increased osmolarity on the ocular surface which causes the common symptoms of itchiness, redness, burning sensation, dryness and sensitivity to light.
The first-line therapy for DES is external augmentation of the tear film with topically administered artificial tear substitutes. Currently, there are numerous formulations on the market attempting to enhance tear film stability; however, many have been found to only temporarily relieve the symptoms of dry eye rather than to heal the ocular surface or treat the underlying cause of the disease.